Every diver knows that small apprehensive feeling as they shuffle forward on their large penguin feet, rocking like a pendulum on the moving deck. Top heavy with hoses and tanks hanging off them they muddle towards the free edge, ready to step forward into the deep blue ocean. Having cleared that hazardous journey, albeit mere metres, they balance themselves precariously on the threshold.
The last checks are performed; regulator entered into mouth, buoyancy aid inflated. Matching time with the surging deck, with one hand covering the face mask and mouth piece whilst the other holding the ancillary hoses, they step forward into the abyss. Clearing the boat they enter into the ocean, the senses are momentarily overwhelmed by the strange new world: Bubbling water cloud their vision and the splashes distorts all sound. But it’s the thrill of cold water felt throughout the body as the blue swallows them all that gives the greatest shock. The tight wetsuit flushes with cold, ocean water, giving the diver a decisive shiver.
The last place I experienced this was in the Andaman Islands, a beautiful set of islands in the tropical waters of the Indian Ocean. The waters though are warm, far warmer than when the legendary diver Jacques Cousteau explored these waters and told the world of the untouched treasures beneath. Sadly, as a result of the warm waters many of the shallow coral reefs have died and are a barren landscape of beige, calcium carbonate skeletons, a mere suggestion of what once was.
Diving here thus means going deeper, to 35 metres, where marine life can still thrive, albeit with the reduced sunlight of the deep. To dive deeper poses many challenges to the diver including nitrogen narcosis, decompression problems and a reduction in air supply. But it also means that you have to be more aware of your body temperature – a cold diver can quickly become a seriously ill diver and it gets a lot colder below. More than once I saw divers signal to their buddies with the international ‘I’m cold’ signs of folded arms rubbing each other preceding a flurry of hand communication and finally their ascent to the surface and the warmer waters above.
Maybe their bodies could not cope with the cold or maybe their wetsuits weren’t thick enough, at any rate they were not insulated enough. In my last post I had flamboyantly broadcasted how keeping warm is really about trapping air, but as the name suggests, wet suits are used in the water and there isn’t much air there. So how do they work?
Firstly, air doesn’t have any super special properties but the three things that make it the ideal insulator are that it is abundant, free and is a gas.
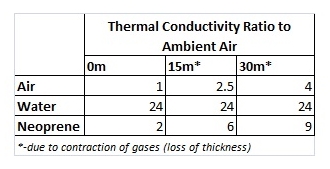
The magic of the wetsuit lies within the neoprene. Neoprene is a plastic foam material that is impregnated with small bubbles of nitrogen gas (like an Aero chocolate bar). Pure nitrogen is slightly better than air at thermal isolation. Putting all this in context, whilst water loses heat an incredible 25 times faster than air, neoprene, due to the amount of air bubbles trapped within it, loses heat at only nine times as fast at 30m depth (only twice as fast on the water surface).
The ingress of water into the suit, which gives that initial uncomfortable shock that all divers experience, is unfortunately a necessary evil. An absolutely skin tight neoprene suit that has no air gaps would be impossible to get on and off (think how thin tights are) and to keep the seals watertight requires some flashy suits that makes for very expensive bits of clothing.
However, we do that too. They’re aptly called dry suits and divers going for extended periods of time underwater or ice diving frequently uses this type of suit to stay warm. Despite being an avid diver and a frequent visitor to the Arctic I have yet combined the two... and I’m aching to try!
Coming back to the point on wetsuits, they rely on trapping a small amount of water between the skin and the neoprene material, keeping that same water there throughout the use.
By trapping only a thin layer of water which is very quickly heated up to body temperature the amount of heat lost would reduce dramatically. In contrast if the trapped water was constantly changing, such as in a loose wetsuit, the body would expend a lot more energy heating up the newly trapped water each time. By transporting the same heated up water with them divers achieve the goal of using the water too as insulation – albeit much less effective as the neoprene covering.
For anyone who has dived, surfed and swam both with and without wetsuits they would quickly attest to how much warmer it is with one.
The wetsuit is an incredible invention that allows us, without ever thinking about it, to dive deep in the Andaman Sea. Half an hour into my last dive on the Andaman Islands when many of the group had already ascended due to cold or low air I came across one of the most majestic and curious of ocean creatures. Magical!
Next: How to stay cool.
If you’re like me and need some equations, see below you science fiends:
There are three types of heat transfer; conduction, convection and radiation. The one that we are talking about most of the time is conduction.
Conduction is a nice long word that basically describes how heat (energy) is transferred between neighbouring molecules, or objects touching each other. The simplest form of understanding this is that if one has more neighbours that are very close together there would be more heat passed (lost). If there isn’t that many neighbours and they are all far apart then there would less heat energy passed on. A solid material has lots of neighbouring molecules all very close together whilst a gas has the exact opposite, with liquids sitting somewhere between.
This property of the material is known as the thermal conductivity.
The other factors that affect heat transfer is described in the conduction equation below.
Heat conduction (per unit time) = (Thermal conductivity) x (Area) x (Temperature difference)/Thickness
It all describes what we all know from common sense. If your jacket (insulation) is thick then you lose less heat, but if you have a larger surface area exposed then you will lose more heat. The final part of the equation suggests that if the difference between temperatures is greater, then more heat is lost, hardly surprising really.
The differences in thermal conductivity are:
Diving deep under water increases the pressure which would lead to the compression of gases, the values for thermal conductivity are given below: